Ingredients by Zaidan
Ref: George Zaidan (2020). Ingredients: The Strange Chemicals of What We Put in Us. Dutton.
___________________________________________________________________________
Summary
An introduction to the chemical composition of the stuff we eat, drink, inhale, and smear on ourselves with a focus on the science, the studies, and the misinformation.
You eat for the e-; you breathe for the O; you drink for the H. Take any one of these away and you die.
The more chemicals something contains, the worse for you it is. This is, in my opinion, wildly illogical. You know what else has thousands of chemicals in it? Iceberg lettuce. So does chicken. And lima beans. By contrast, cyanide has exactly one chemical in it. It is one chemical, and a very simple one at that—and it’s deadly.
Any time you read about an association between two things, two questions should pop into your head: Is this association legit? If the two things are legitimately associated, the next logical question is: Does one of the things cause the other? In other words: Is this association causal?
All the people involved in scientific studies—the participants, the surgeon general’s committee members, the scientists puzzling out the mechanism—and all the animals who died in service of the cause did something pretty incredible. They built a bridge from the Land of Not Knowing, shrouded in mist and shadow, to the Land of Almost Certainly Knowing. This bridge is built from thousands of experiments and studies, each a dense brick, supporting and being supported by others, without holes between them, together spanning the full width of the Gulf of Ignorance. Instead of something cool like BRIDGE OF TRUTH, scientists decided to name these bridges “theories.”
I’m not a fan of the word theory, for two reasons: It has two exactly opposing meanings in science vs. English (solid vs. flimsy), and the English definition has already won.
___________________________________________________________________________
Studies & Experiments
Nocebo Effect: if you expect something to feel like crap, it will.
Randomized Controlled Trial: A type of experiment where you require groups of people to do different things.
Prospective Cohort Study: A type of experiment in which you recruit a bunch of people and check in with them regularly for years, but you don’t require them to change their diet or behavior in any way.
Long-term prospective cohort study: You enroll a bunch of people, ask them lots of questions about how they live their lives, follow them over a long period of time, and record what diseases they get. What pops out of these studies is an association (also called a correlation).
Adversarial Collaboration: When scientists work together on the exact topic they disagree so violently on.
Confused Associations: Legitimate but noncausal associations that are caused by some other hidden factor.
GRADE System: A systematic way to look at a bunch of evidence and decide how good (or bad) it is.
Preregistration: where you tell the world exactly which variables you’re going to test—and exactly how you’re going to analyze the data—before you enroll a single participant in your study.
Issues with Scientific Studies
Pothole #1: Fraud.
Pothole #2: Basic Mathematical Mess-ups
Pothole #3: Procedural Problems
Pothole #4: Random Chance
Pothole #5: Statistical Skulduggery, including p-hacking
Cochrane Database of Systematic Reviews: Reviews studies on many foods, interventions, and health outcomes, and they try to detect and call out the chicanery that makes its way into the scientific literature.
If your neighbor’s risk of getting mauled by a mountain lion is 25% and yours is 40%, then your relative risk vs. your neighbor’s is 40/25 = 1.6, which means: You’re 1.6 times as screwed as your neighbor. You’re 160% as screwed as your neighbor. You’re 60% more screwed than your neighbor.
___________________________________________________________________________
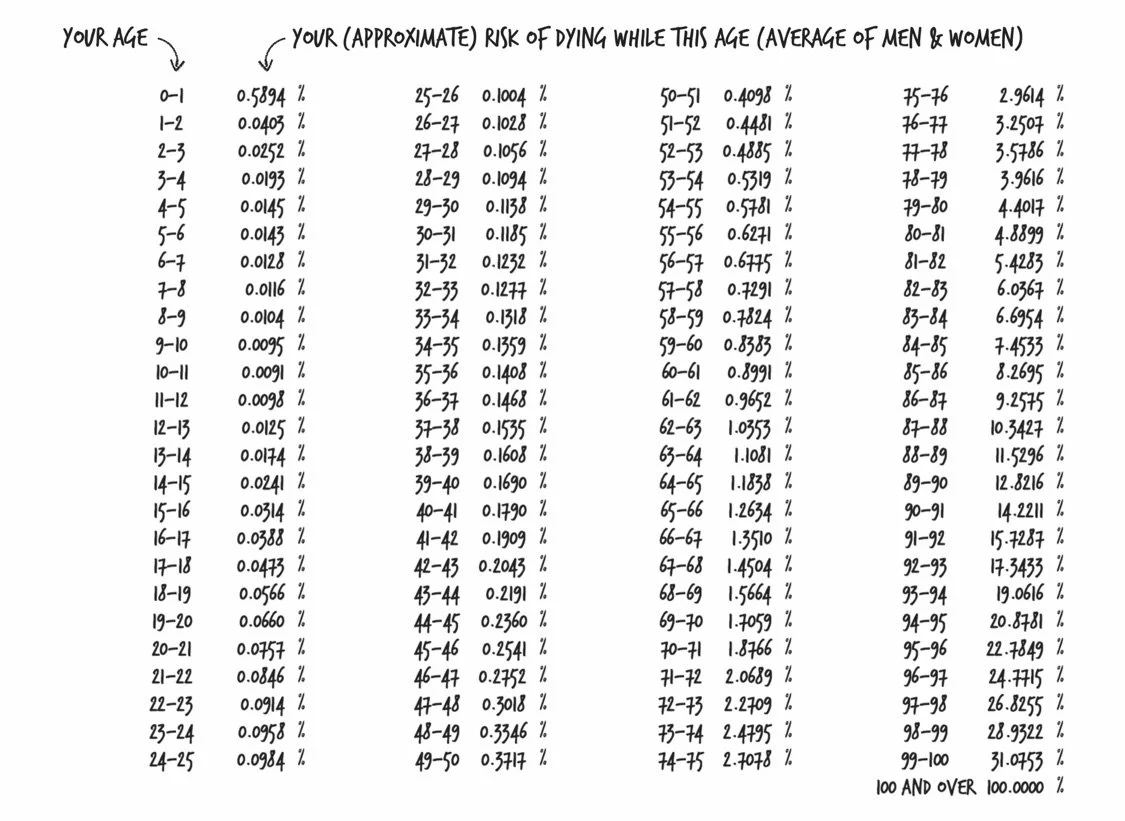
Longevity
If you compare people in the absolute worst groups in every lifestyle choice to those in the absolute best, the life expectancy difference is about 20 years!
Using data from the CDC, Willett’s group estimated that only 0.14% of Americans were in the worst health group and 0.29% were in the best.
Accident Hump: At its peak, the risk of death ratio hits 2.85, meaning men are 285% as likely to die as women. Unsurprisingly, this happens at age 22.
Nutrition: People who ate the “healthiest” diet had a four- to five-year-longer life expectancy than people who ate the least healthy diet.
Ultra-Processed Foods
In the U.S., over 58% of our calories come from ultra-processed foods and increase your risk for basically every disease, but especially diabetes, heart disease, and cancer.
In an 8,000-person trial in Spain with about nine years of follow-up. The authors found that people who ate about 4x as much ultra-processed food had a 26% higher risk of becoming overweight or obese over the course of nine years.
Researchers in France recruited over 100,000 people and followed them for an average of five years, diagnosing cases of cancer as they came up. They found that people who consumed, on average, about 4 times as much ultra-processed food had a roughly 23% higher risk of developing any cancer.
Obesity: People with a BMI of over 35 had a life span between 4-6 years shorter than those with a BMI of 23 to 25. People with a BMI of 25 to 30 had a life span of only a year shorter than those with a BMI of 23 to 25.
Alcohol: People who didn’t drink at all and people who drank 30 g of alcohol per day had roughly the same life span—about two years shorter—than people who drank between 5-15 g per day.
Exercise: People who did 3.5 hours or more per week of “moderate or vigorous physical activity” lived roughly eight years longer than those who did no exercise.
Money: In the USA, people aged 40-76 who are in the top 1% by income (as measured by the Social Security Administration) live 10-15 years longer than people in the bottom 1%.
Hall estimated that the cost of 2,000 calories was about $15 for ultra-processed food vs. $22 for minimally processed food. That’s a difference of about $2,500 per person per year. If you’ve got a family of four, that’s ten grand a year. So, for many Americans cutting out ultra-processed food is not a choice; it’s a luxury.
___________________________________________________________________________
Nutrition
Diets: Really just two lists- a good list of foods you should eat and a bad list of foods you shouldn’t.
Most food starts with (or includes) murder. Almost everything you have ever eaten or will ever eat was once a living, respiring thing, or part of a living thing.
Nutritional epidemiology is mostly based on long-term prospective cohort studies.
The Healthiest known Diets: lots of fruits, veggies, nuts, whole grains, polyunsaturated fatty acids, and long-chain omega-3 fatty acids, and very little or no processed meat, red meat, sugary drinks, trans fat, and salt.
Scurvy: the scourge of the sea; Symptoms start gradually—fatigue, aching joints, painful muscles—but got progressively more terrifying. Splotches of blood pooled beneath the skin. Gums bled easily. Body hairs coiled like snakes. Some historians estimate that more than two million sailors died this way between roughly 1500-1850.
Processed Foods: a whole bunch of variables tied together in one convenient package: high energy density (amount of calories per gram), low volume, delicious flavor, factory production, high salt level, and so on.
NOVA Group 4: Ultra-processed foods; not modified foods but formulations made mostly or entirely from substances derived from foods and additives, with little if any intact Group 1 food. Ultra-processed foods include additives that aren’t found in other foods, including flavorings, dyes, and this delicious-sounding list of stuff: “carbonating, firming, bulking and anti-bulking, de-foaming, anti-caking and glazing agents, emulsifiers, sequestrants and humectants.”
___________________________________________________________________________
Vitamins, Minerals, Proteins, Amino’s
Vitamin A (retin-): An antioxidant used in various skin formulations and shown to prevents wrinkles. Unfortunately, too much vitamin A can cause liver damage, brittle nails, and hair loss, and can contribute to osteoporosis in older adults and skeletal birth defects in developing fetuses. Vitamin A’s toxicity is the fact that, when spread over the skin and hit with UV photons, it significantly increased the number of skin tumors and lesions in mice.
Vitamin B1: Comprised of 35 atoms. A severe lack of it can cause beriberi.
Vitamin C: Comprised of only 20 atoms, Vitamin C helps your gut absorb Fe by giving them an extra electron each, helps protect DNA, and produces collagen, a rigid triple-helical protein that makes up between a quarter and a third of all protein in the body.
Humans, fruit bats, and guinea pigs cannot make their own vitamin C.
Humans require ~10mg daily.
Vitamin D: Comprised of 72 atoms. A severe lack of it in kids causes rickets.
Rickets: A painful softening of your bones that can leave you bowlegged and stunt your growth.
Amino Acids: The building blocks of protein, of which there are roughly 20 different amino acids in all of nature. The human body—and the bodies of most animals, can produce about half of these.
ATP: Your blood carries O to pretty much every cell in your body, where little mini-cell-like things called mitochondria use it to produce a molecule called adenosine triphosphate, or ATP. You can think of ATP as a molecular AAA battery. These AAA batteries are the main source of energy for most cells in your body, so most cells have lots of mitochondria in them. O is critical in the very last step of AAA battery production. Electrons (from the chemical bonds in your food) are smashed into an O molecule (from the air you breathe), along with two hydrogen ions (probably from the water you drink), to form a molecule of water and simultaneously power the reaction that makes that AAA battery.
___________________________________________________________________________
Food Storage
Canning: Denies microbes their access to O.
Clostridium botulinum thrive in O-free environments, which means that once foods with a pH higher than 4.6 are canned, they have to be heated to a specific temperature and held there long enough so that the probability of finding C. botulinum in a can is one in a billion.
Fermentation: Encourages the growth of good microbes like lactobacillus; responsible for yogurt, cheese, sour cream, beer, wine, vinegar, sauerkraut, kimchee, bread, and countless other foods.
Bacchanalia turns a perfectly happy microbial home like milk with a pH of 6.5-ish into a corrosive hellswamp, 100x more acidic than milk and absolutely unlivable to the vast majority of all other microbes, including and especially the ones that make us sick. This corrosive hellswamp goes by the common name of “yogurt.”
Freezing: Slows down all molecular motion—and therefore all life—and frozen water takes on a highly rigid, crystalline structure that is capable of bursting cells and incapable of supporting microbial growth.
Fresh fruits and vegetables can be chilled to slow down molecular motion and thus spoilage.
Drying: Submersion of food into a complex mixture of liquid fats heated to about 302°F (150°C) to boil off water.
Manothermosonication: Used to preserve milk and Orange Juice.
Freeze-drying: Get a strong vacuum pump, some alcohol and dry ice, and some leakproof piping and flasks. Freeze the thing you want to freeze-dry, then put it in a flask. Connect the flask to some pipe, then connect the other end to a second flask. Dunk the second flask into an alcohol/dry-ice bath. Connect the second flask to a vacuum pump. Turn on the pump, and let run for at least twelve hours. A few hours in, heat the flask gently with one of those red lightbulbs that keeps you warm after a shower. Wait a few more hours, and finally . . . Enjoy your NASA ice cream.
The vacuum pump lowers the pressure to almost zero, which causes the frozen water in the ice cream to start to evaporate—without melting. Heat from the light helps this process along. As the water vapor enters the second flask, it freezes. Eventually you end up with freezing cold, bone-dry food. Essentially what you’re doing is using low pressure, extreme cold, and gentle heat to remove solid water (ice) out of a frozen food without melting the food first.
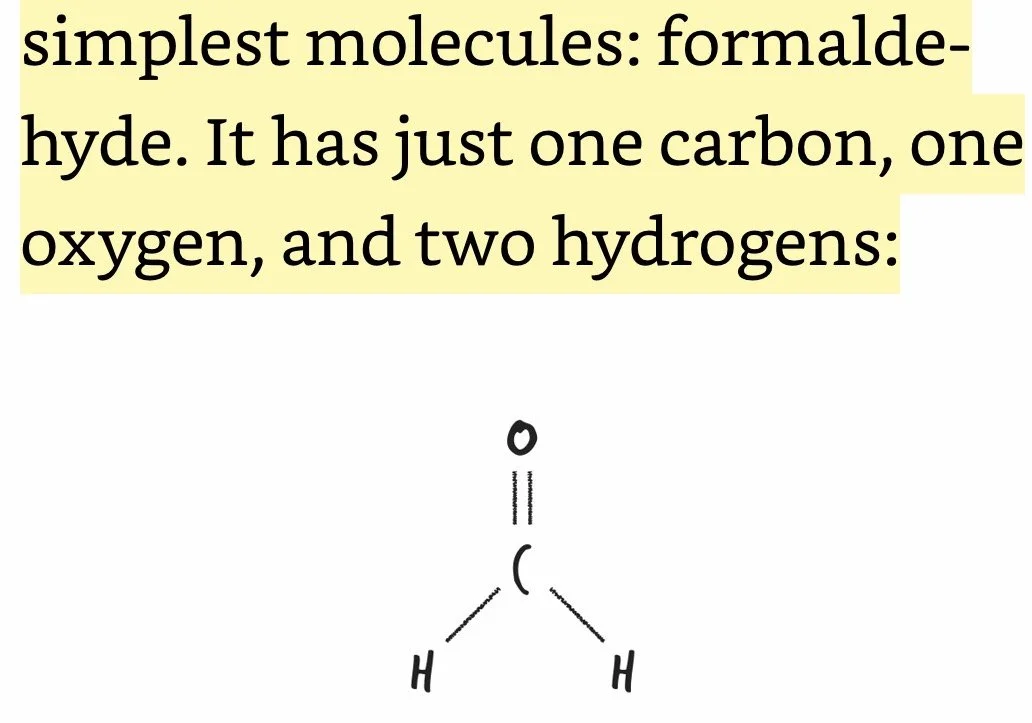
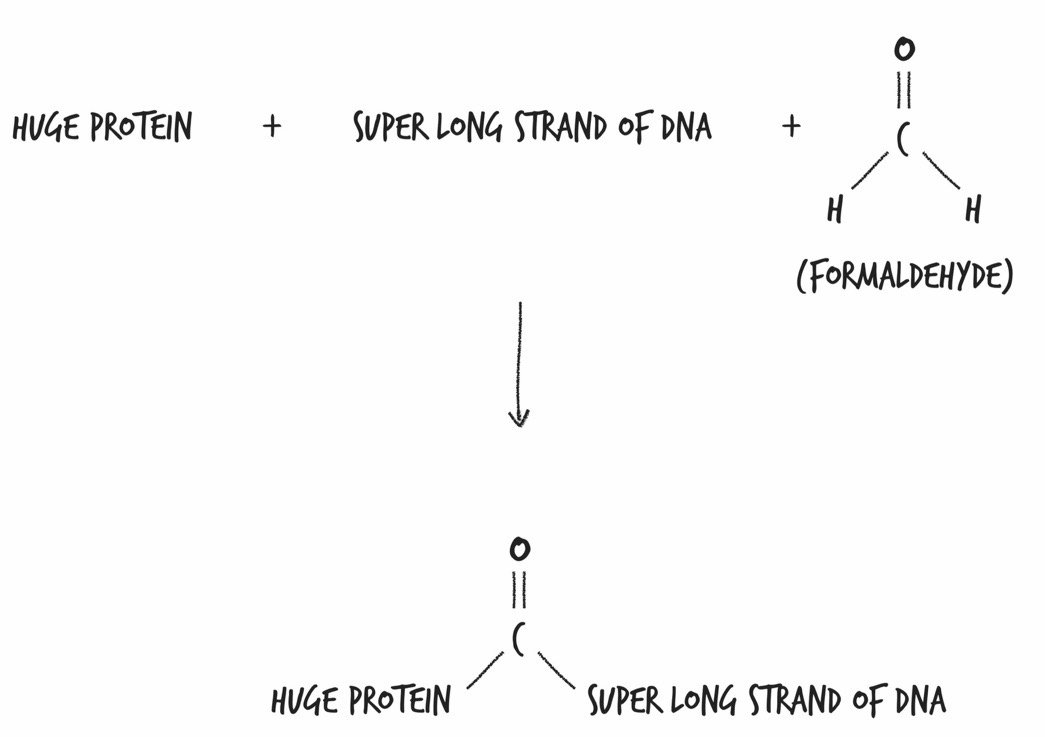
Formaldehyde: Comprised of one C, one O, and two H’s; formaldehyde comes as close as chemically possible to stopping the motion of life within each and every one of her cells, as well as within the cells of all the organisms that were chowing down on her.
___________________________________________________________________________
Photons & Sunscreens
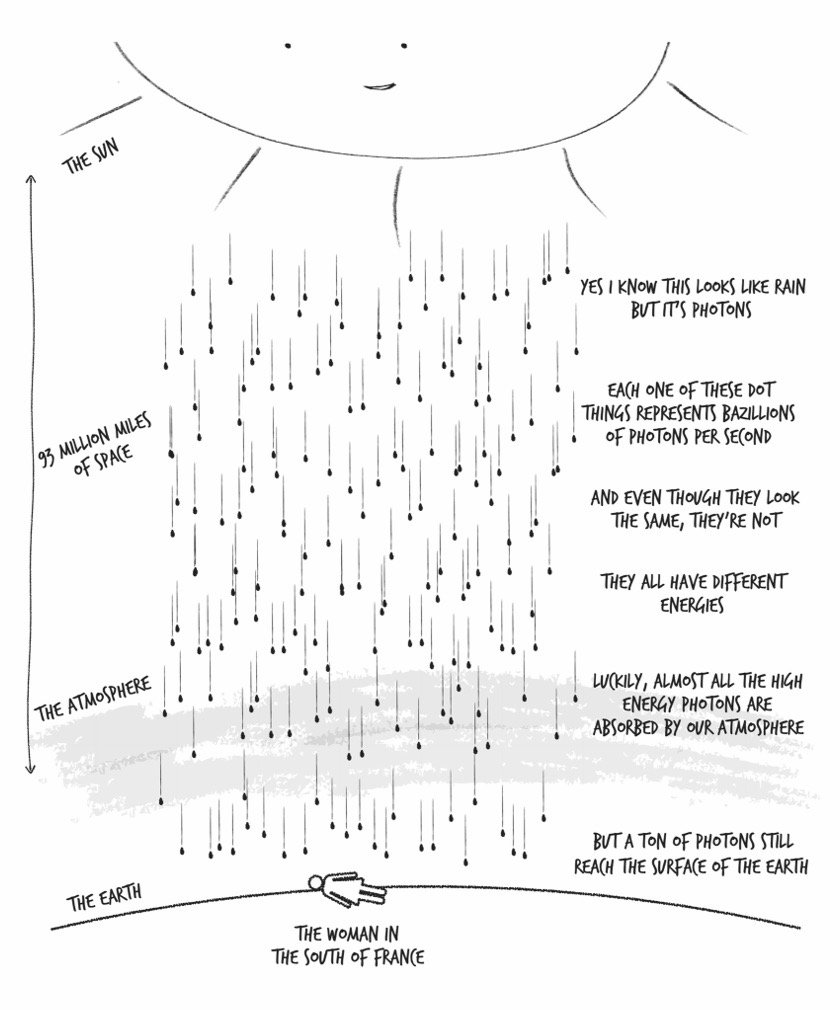
Vision: Eyes are extremely sensitive photon detectors, and what you know as “light” is actually an unimaginably large number of photons on the last leg of their journey from the sun, bouncing off everything around you and crashing into photon-detecting proteins in your retina. This collision generates an electrical signal that your brain interprets as whatever you happen to be looking at.
Our eyes can detect photons within only a very narrow energy range: from 2.8e-19 to 5.2e-19 joules. But the energy range of photons emitted from the sun is much wider: roughly 2e-28 to 2e-16 joules. Most of these, though, are helpfully absorbed by that thin layer of O3 molecules in the atmosphere.
Infrared (IR) Light: Photons that make your skin molecules dance (like hot water does). If you get hit by enough IR photons, a wide array of bad things happen. Cells explode. Proteins coagulate and become useless. Eventually the water boils off and the solids combust, forming gases and C, which you know as the charring on your steak.
Ultraviolet (UV) Light: High Energy (5.2-6.8e-19 J) photons cause photosynthesis in the skin producing Vitamin D.
The outermost layer of your skin contains lots of 7-Dehydrocholesterol, or 7-DHC, one of cholesterol’s much less famous chemical cousins. 7-DHC, when hit by these particular photons, changes into a molecule called previtamin D3, setting off a chain of events that produces the active form of vitamin D.
Photons penetrate deeper into human skin than you might think: at least a mm but possibly more, depending on your skin tone and the exact energy of the photon.
Sunburn: Interactions of photons, the cells and body, and the cells and molecules inside those cells: DNA, proteins, sugars, fats, cholesterol, and water, to name a few. When these photons hit electrons in all those molecules, they make them move in all kinds of different ways. Whole molecules spin around and/or pairs of atoms within the molecules get stretched further apart, compressed closer together, or bent, rocked, scissored, wagged, or twisted relative to a third atom.
Sunscreen: A creamy white splooge that you spread over your body to convert the potentially DNA-damaging energy from hundreds of million septillions of UV photons per second into mostly harmless heat.
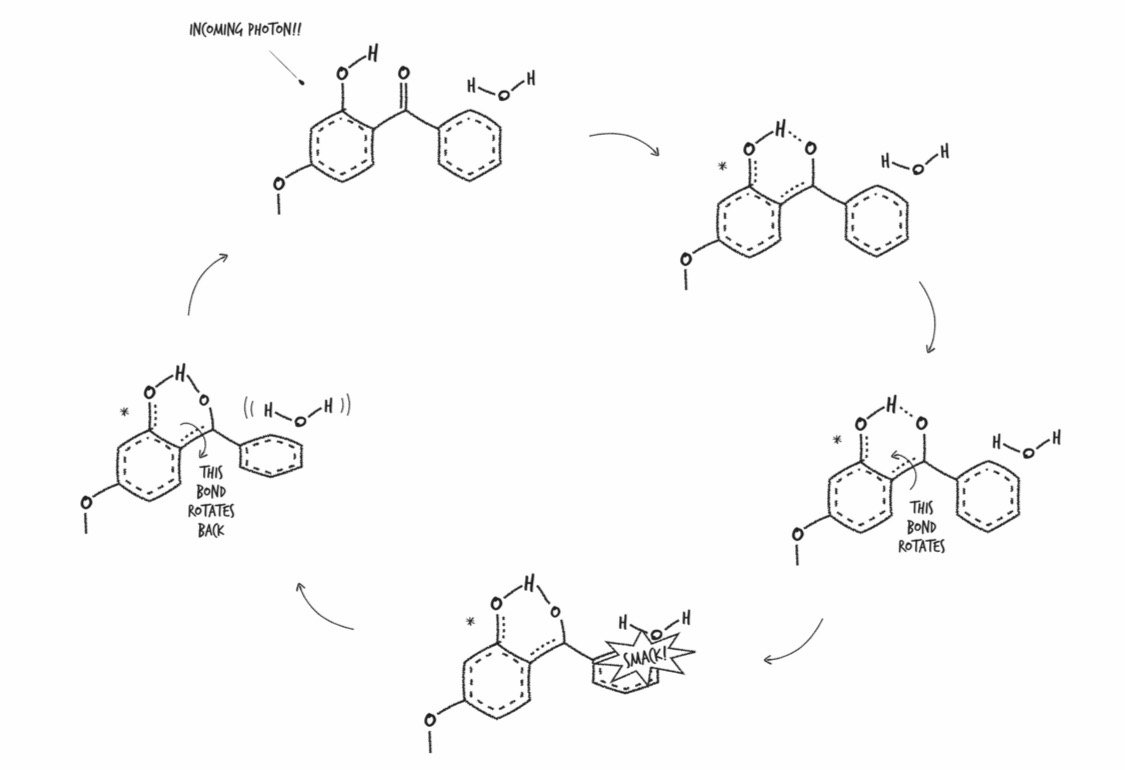
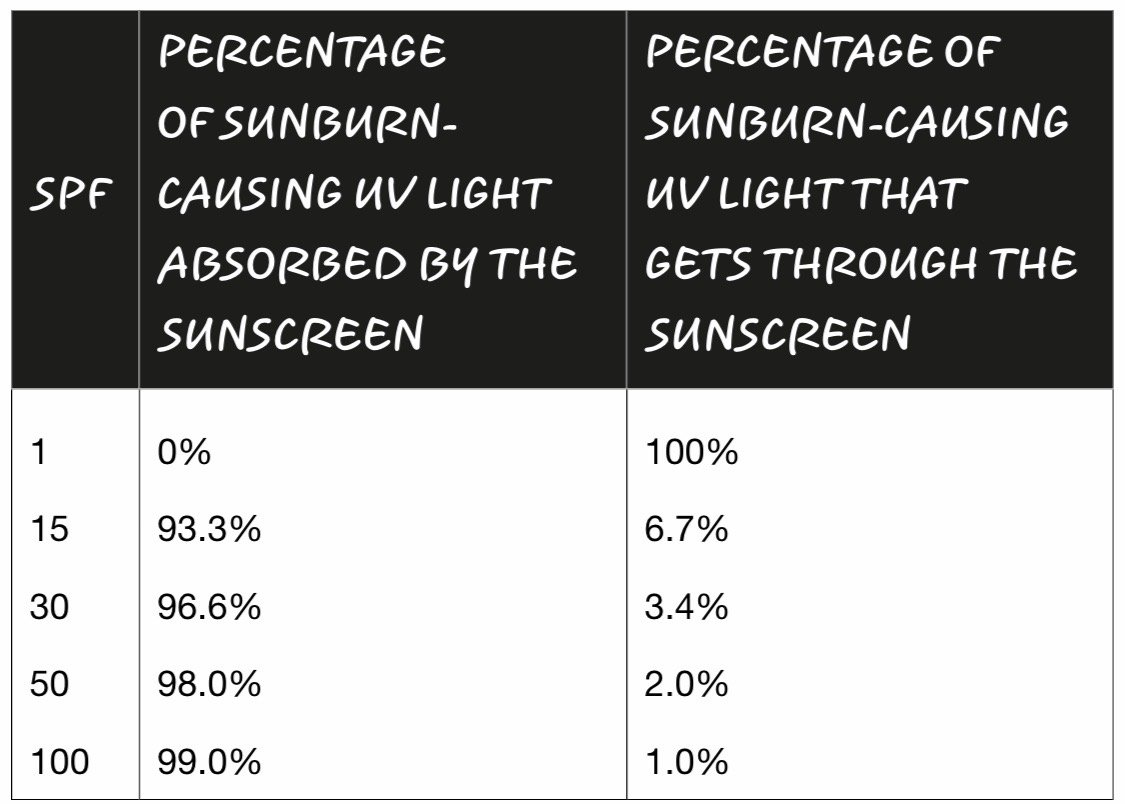
When a UV photon from the sun hits a molecule of oxybenzone on your skin, it sets off a somewhat complicated chain of events. First, the photon crashes into an oxybenzone molecule, putting it in an excited state, which just means it has more energy than it did before. Its convert-UV-photon-to-heat cycle happens fast: it takes roughly ten-trillionths of a second for a molecule of oxybenzone to get back to the way it was. This means that one molecule of oxybenzone can absorb about 90,000,000,000 UV photons per second. If you apply the FDA-recommended amount of SPF 30 sunscreen, what you’re doing is enhancing your skin’s ability to harmlessly dissipate the energy from well over 700,000,000,000,000,000,000,000,000,000,000 UV photons crashing into you per second.
SPF 50 lets in roughly half the amount of sunburn-causing UV energy as the SPF 25 sunscreen.
Common Sunscreen Ingredients
Most common active ingredients in sunscreen: oxybenzone, octinoxate, octocrylene, zinc oxide, and titanium dioxide.
Homosalate: Can enhance the absorption of herbicides through your skin and into your blood.
Octyl methoxycinnamate: Has been shown to decrease motor activity in female rat babies.
4-methylbenzylidene camphor: Has been shown to reduce female rats’ sex drive and impair early muscular and brain development.
Octocrylene: Messes with the expression of genes related to development and metabolism in the brains of zebrafish.
Zn-oxide & Ti-dioxide nanoparticles: have been shown to affect spatial cognition in rats, impair learning and memory and increase reactive oxygen species in mice, decrease acetylcholine esterase activity in fish, decrease brain weight in honeybees, reduce cell viability in human brain cells, increase oxidative damage to the hippocampi of male rats, and decrease hatch time and increase malformation rate in zebrafish.
Parabens: have been shown to disrupt the endocrine system, which could increase your risk of reproductive toxicity.
Oxybenzone, benzophenone-4, avobenzone, octyl methoxycinnamate, octisalate,
Methylisothiazolinone: Named Allergen of the year by the American Contact Dermatitis Society in 2013.
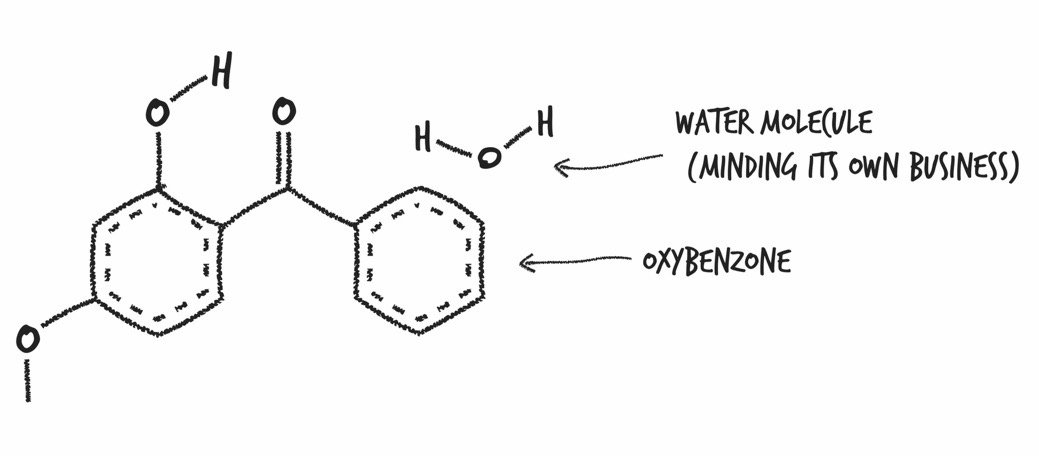
Oxybenzone (aka benzophenone-3): Can soak through your skin and get into your pee, breast milk, and bloodstream, and once there, mimic your hormones. Animals exposed to oxybenzone and octinoxate, another UV absorber, were found to have lower sperm counts and higher sperm abnormalities. Female mice exposed to oxybenzone had messed up menstrual cycles. A recent study found that adolescent boys with higher oxybenzone levels had significantly lower testosterone levels.
Oxybenzone has been shown to damage the DNA of coral larvae and cause reef bleaching and death.
8 of 13 UV filters (sunscreens) approved for use in the USA have been shown to affect Ca signaling in male sperm cells.
___________________________________________________________________________
DNA & Cancer
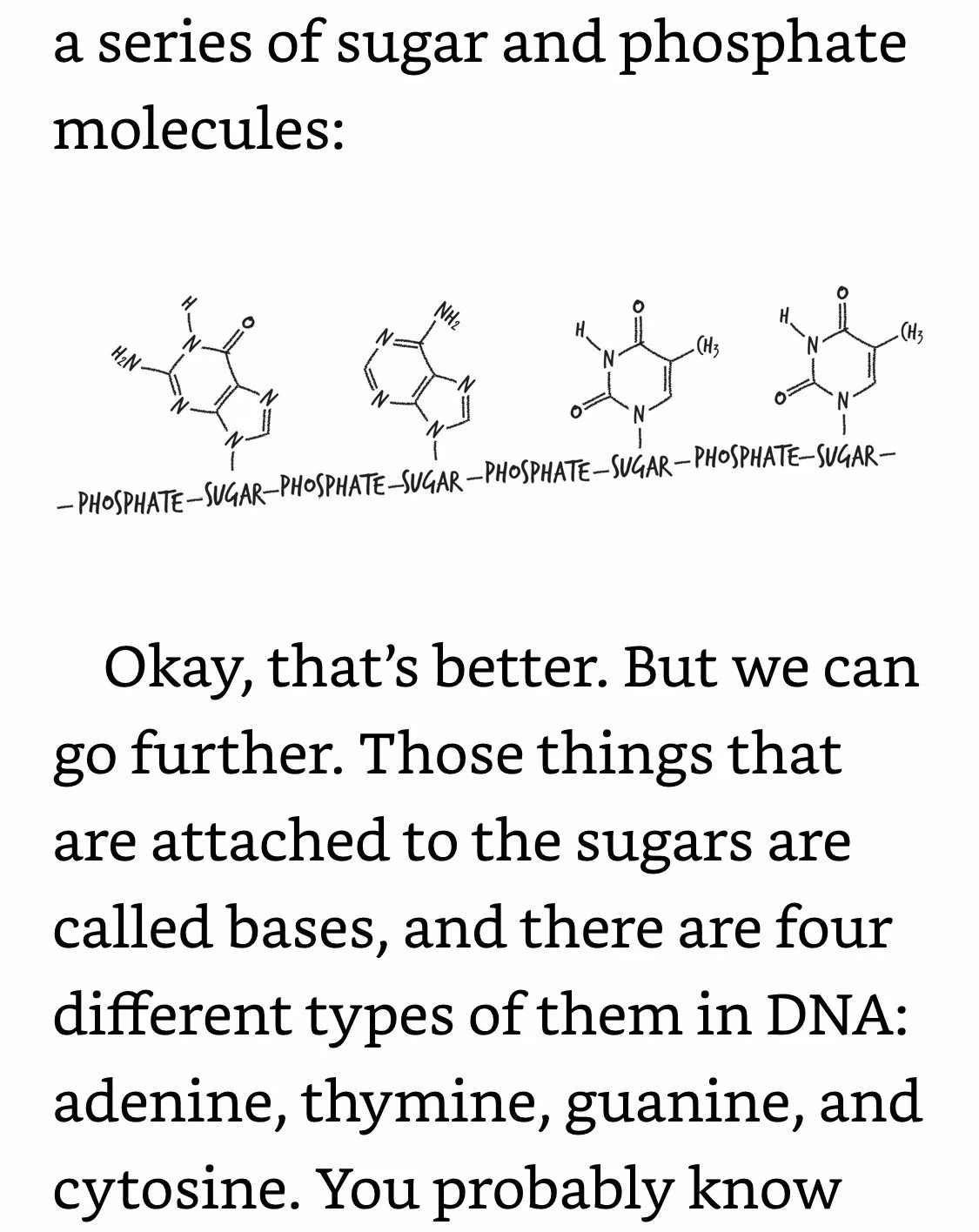
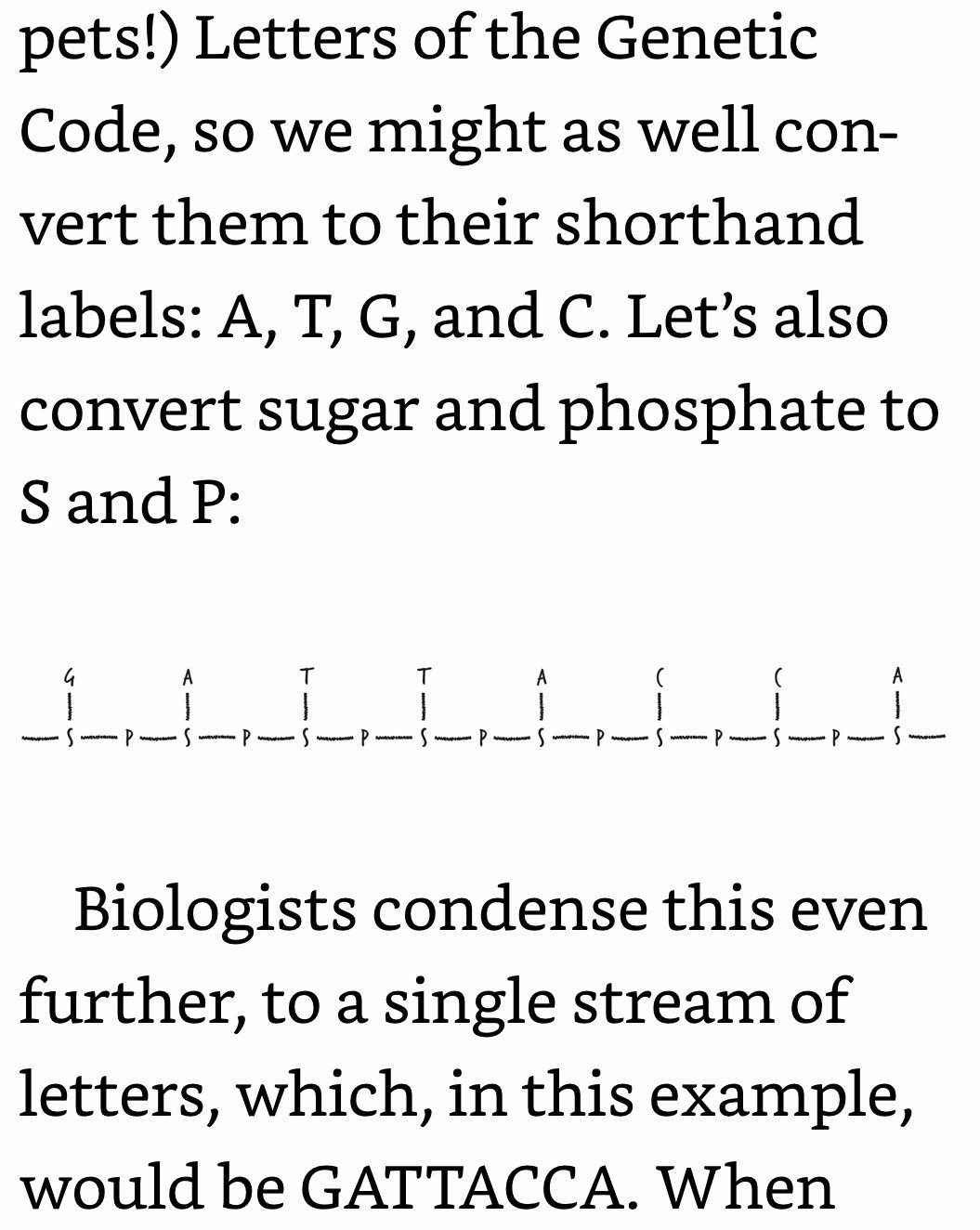
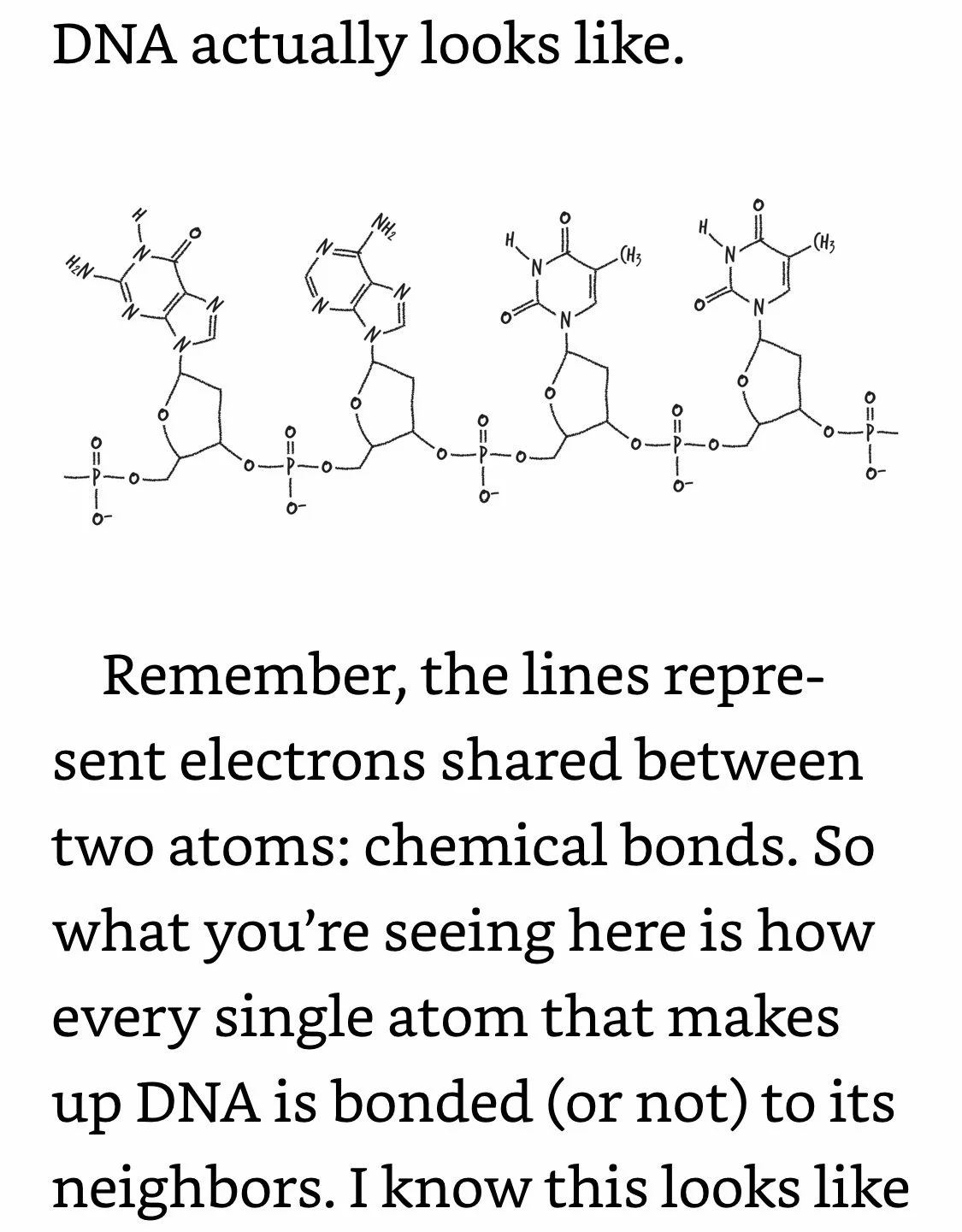
DNA Coding: Phosphates are bonded only to sugars. Letters are bonded only to sugars. Letters are not bonded to adjacent letters. Your DNA is an exquisitely intricate coding system, but the code itself is dependent on how DNA’s different components are bonded (or not bonded) to each other. And since DNA’s bonds are electrons, what that means is that DNA can’t do its job unless its electrons are in the right places.
Mutations: A change in the genetic code, the blueprint the cell uses to live its life. Most mutations don’t do anything at all. Only about 1% of your genome actually codes for a protein, so if a mutation happens elsewhere, it’s probably harmless.
Genes
KRAS: push cells to grow faster (KRAS).
TP53: Prevent cells from shutting themselves down if they get out of control.
Ribosome: The molecular machine responsible for assembling proteins based on the sequence of your DNA. Ribosomes are pretty damn big, by cellular standards: they’re made up of 79 proteins and a few thousand-unit-long chains of nucleic acids (RNA).
Cancer: Carcinogens enter your body, are activated by cytochrome P450 and chemically react with DNA, this disrupts important processes like DNA copying, so your body tries to fix the damage which occasionally results in mutations, mutations accumulate in your DNA, enough mutations in genes that control cell growth can put cells on the path to cancer.
Skin Cancer
The Risk of skin cancer in white skin is between 1,600% and 6,300% as high as in black skin. Why? Melanin.
Melanin: A molecule your body produces and distributes throughout your skin; it absorbs UV photons preventing them from damaging your DNA. The more melanin in your skin, the less DNA damage. Melanin also absorbs visible light, which means the more melanin in your skin, the darker it is.
Most of the time DNA is hit by photons, the excited electrons just come back down to their non-excited state, and your DNA remains chemically the same as it was before.
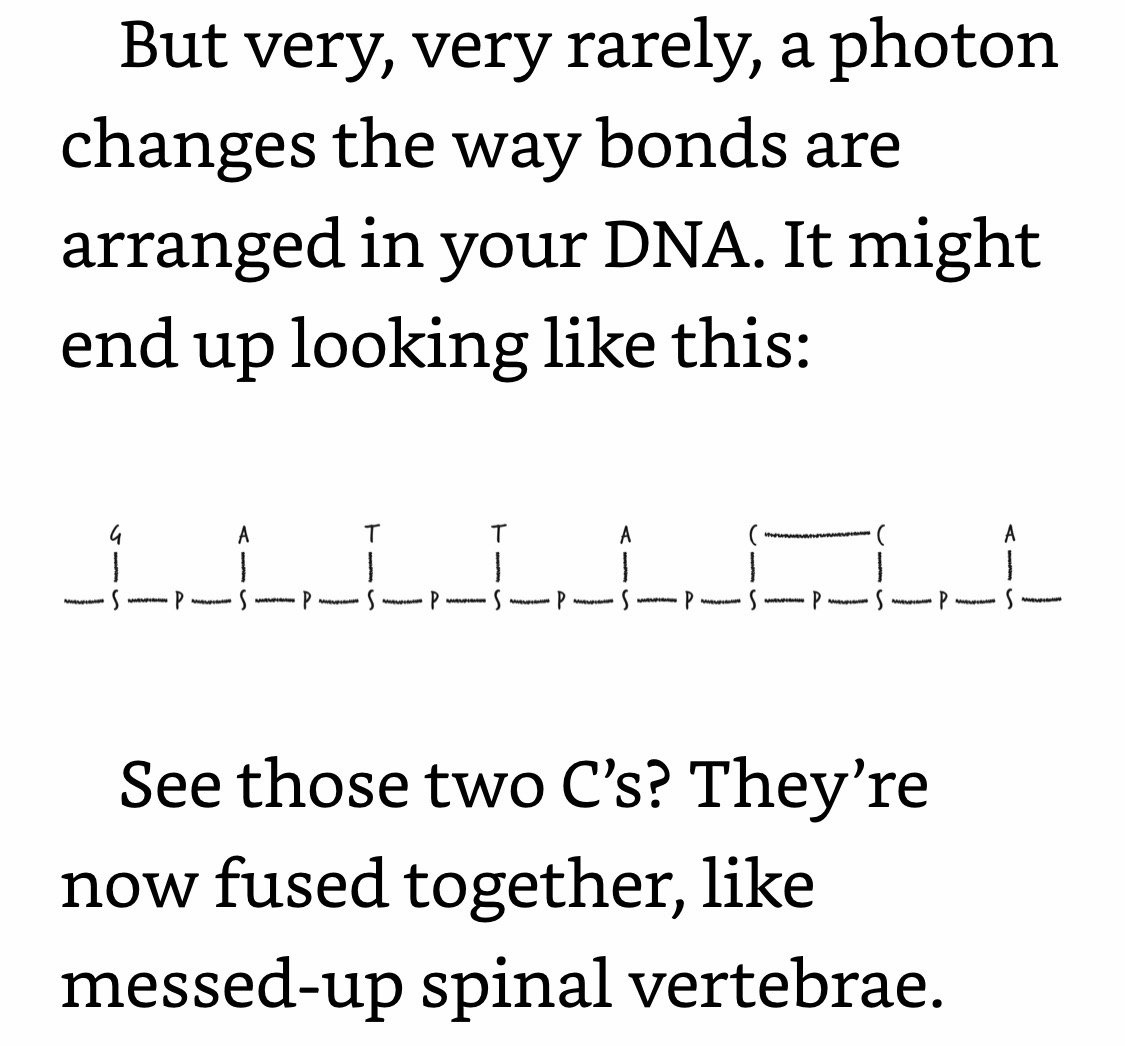
Most of the time, your cell detects this fused-C problem and repairs it. You go on with your life as if nothing happened. Occasionally, the repair effort fails catastrophically, in which case the cell kills itself. This is the medium-case scenario. The worst-case scenario—which is relatively rare—is that at some point during the repair or even cell division process, the cell copies its DNA wrong. So, a sequence like “GATTACCA” might become “GATTATTA.” In other words, the worst-case scenario is that a mutation might creep into the DNA sequence.
Your body can detect and fix a fused-C. But once that fused-C becomes, say, a TT, your body can no longer detect or fix it, because that mutation is chemically healthy DNA (even though it’s informationally diseased).
Two basic types of skin cancer
Melanoma: Accounts for a minority of skin cancer cases but causes most deaths.
Non-Melanoma: Most skin cancers.
Squamous Cell Carcinoma (SCC)
Basal Cell Carcinoma (BCC): Very slow growing and rarely metastasizes
___________________________________________________________________________
Smoking
The prospective cohort studies on smoking found an association between heavy smoking and a 1,000+% increased risk of getting lung cancer.
Nicotine: A defensive mechanism made by tobacco plants to kill insects, a natural pesticide.
The lethal oral dose of nicotine is ~10 mg per kg of body weight. That means that a 30 ml bottle of high-nicotine e-liquid contains enough nicotine to kill a fully grown adult, and more than enough to kill a toddler.
E-cigarette: A metal coil heats up an e-liquid to about 302°F to 662°F (150°C to 350°C), which generates a mist. Regular cigarettes burn much hotter: 1,472°F (800°C) or higher. Because the temperature is much lower in an e-cigarette, and because e-liquid is chemically much simpler than tobacco, the number of chemicals in e-cig vape is almost certainly much lower than in regular cigarette smoke.
The amount of formaldehyde in e-cigarette mist is roughly one-tenth the amount in regular cigarette smoke, and the amount of NNK (the potent lung carcinogen) in e-cigarette mist is roughly one-fortieth the amount in regular cigarette smoke.
Drug Patches: work by putting the drug in close contact with your skin; it slowly enters your body and makes its way into your bloodstream. Unfortunately, when your skin gets warmer—as it would in the sun—the amount of fentanyl (or any drug) that can diffuse into your body increases.
If you were to compare an actual smoker’s lung with a nonsmoker’s lung under the microscope, you would see lots of cells called macrophages in both lungs. These cells are part of your immune system and basically gobble up any foreign material—including smoke particles—to try and prevent them from doing damage. But in the smoker’s lung, depending on how long the person had been smoking, the macrophages would look yellow, brown, or even black. This is because smoke particles are chemically difficult to break down, so macrophages store them in little compartments within themselves.
___________________________________________________________________________
Plants
Plant Food
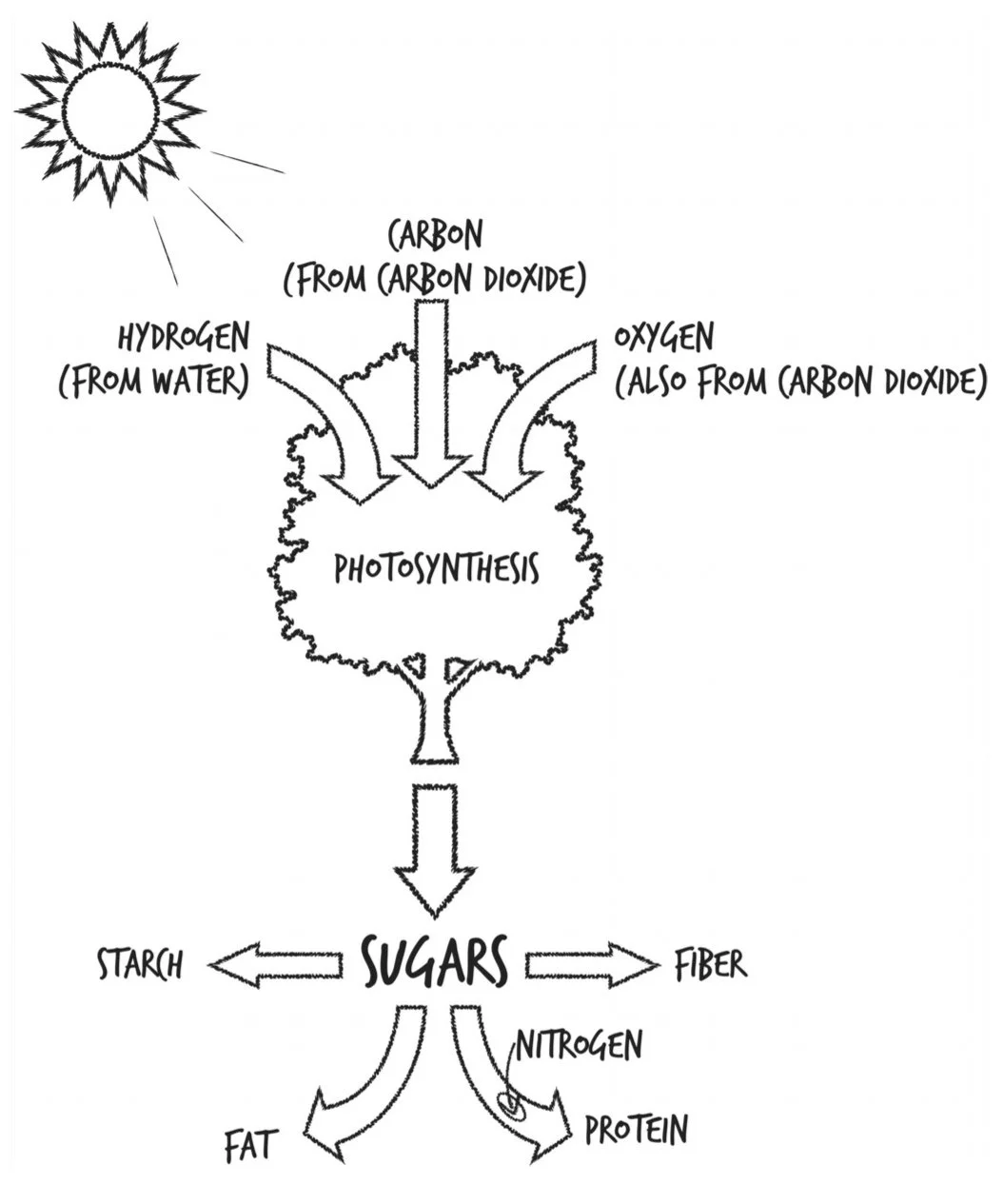
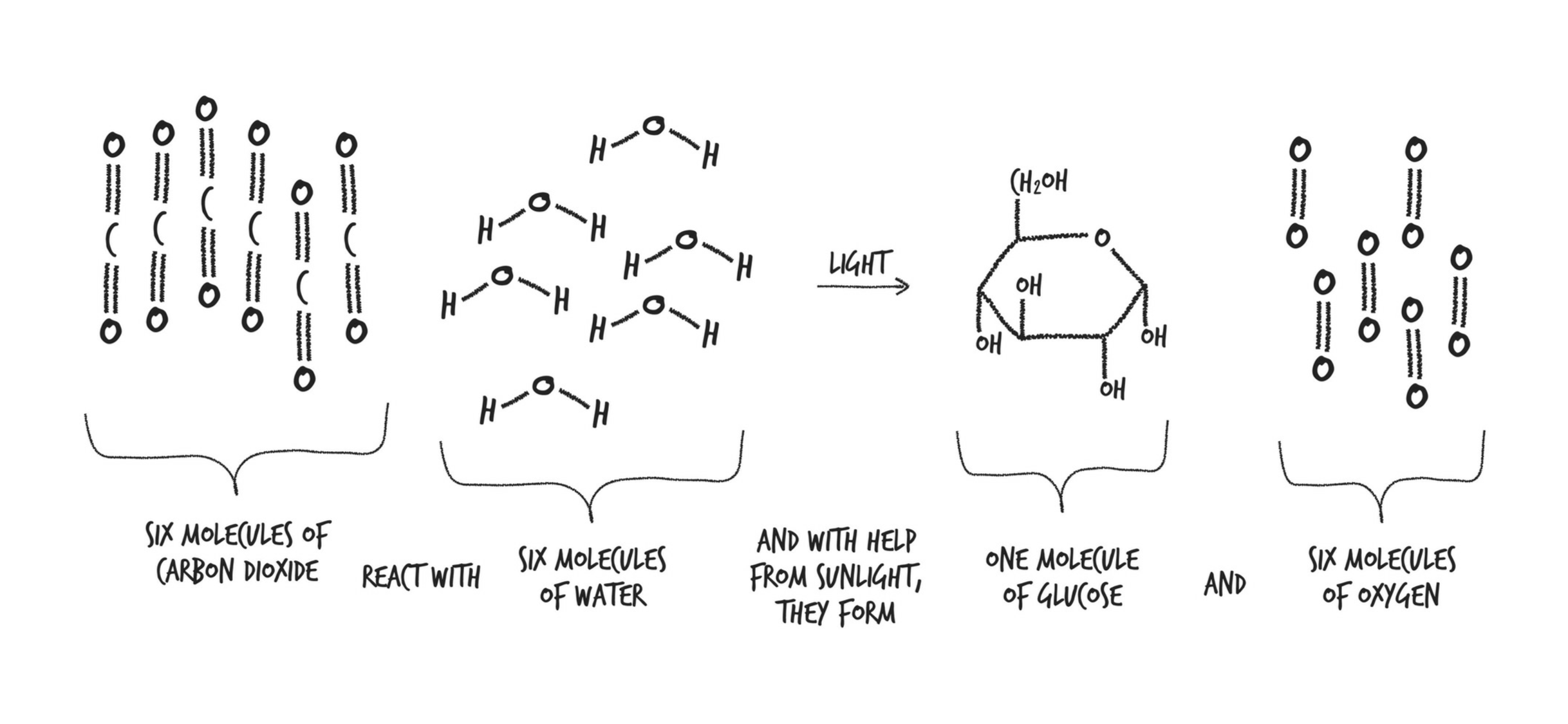
Photosynthesis: takes C, H, & O and turns them into sugars. Plants burn those sugars for energy immediately, but they also store them for later as starch or fat.
Three of our most important food groups—sugars, starches, and fats—are all made from the same three elements harnessed by photosynthesis.
Glucose: The glucose plants make is used in all kinds of ways: It’s burned for energy in the same way we humans burn glucose for energy; it’s made into sucrose (exactly the same as the sugar in your pantry); it’s turned into starch and stored for the winter; it’s turned into cellulose, which is used to build the plant
Even under average conditions, a plant can make about 800mg of sugar per medium-size leaf per day. An oak tree can make 25kg of glucose every single day.
If you dissolve two teaspoons of sugar in a cup of tea or coffee, you’re making roughly a 3.3% sugar solution. To most of us, this tastes pretty sweet. A can of Coke is about a 10% sugar solution. Plant sap starts at 10% and goes up to 50%.
Plants also make protein using N taken in via their roots from the soil. Other plants partner with microbes that can pull N gas out of the atmosphere and make ammonia (NH3), which the plants can then incorporate into themselves as proteins, vitamins, and DNA.
Where do they store all this food- N from the soil and C, H, and O from the atmosphere? They build themselves out of it.
Plant Structure
Xylem: Transports water from the roots up to the rest of the plant.
Phloem: Transports sugar from the leaves to everywhere else. Phloem is a complicated tissue, but the key components are called sieve tubes. Sieve tubes are basically pipes made of single living cells, laid end-to-end like sections of an oil pipeline; the joints are shot through with holes, like the sieve in your kitchen. Each section, called a sieve element, is only a few hundred millionths of a meter long. In leaves, sieve elements are roughly 10 millionths of a meter wide. Plants can pressurize their sieve tubes to about 145 psi!
Plant Defense
Resin: When insects chew up conifers, the trees respond by oozing out a resin dissolved in turpentine at the site of the injury. The turpentine evaporates (carrying some delightfully smelly molecules to your nose), leaving the hardened resin behind, sealing up the wound and forming what we know as “amber.” Some plants store resin under so much pressure that it can shoot out almost five feet.
Cyanide: made of only two atoms: one C and one N and extremely toxic. I weigh about 160 pounds; one-tenth of a gram of cyanide would probably kill me. Half a gram—about the weight of a single regular-size metal paper clip—definitely would.
Instead of making pure cyanide, the plants attach cyanide to a harmless sugar molecule, making what’s called a cyanogenic glycoside.
Cyanide is such an effective poison that it’s found in over 2,500 different plant species.
Rhodanese: An enzyme that helps the body detect Cyanide.
Andrussow Process: Production of Cyanide in which methane and ammonia are burned in O at over 2,000°F (1,093°C) in the presence of platinum.
Tannins: Relatively large molecules, made of tens, hundreds, or even thousands of atoms. Instead of preventing mitochondria from using O, tannins bind to the proteins in food, preventing them from being digested. So, unfortunate tannin-eating mammals just poop out those valuable proteins.
It takes tannin levels of 20% or higher to kill.
Many different species, including moose, beavers, mule deer, and black bears, produce proteins in their saliva that sponge up the tannins, preventing them from binding to the food proteins the animal is trying to digest.
Alkaloids: Made by ~18% of plants, in high doses Alkaloids can shut down your nervous or respiratory systems. In low doses, some alkaloids can be extremely useful drugs.
Ricin: A Ribosome Inactivating Protein; Ricin removes a single nucleic acid from the ribosome, which completely and irreversibly inactivates the entire thing. And then it keeps on going, clipping other ribosomes’ wings, inactivating more than a thousand per minute. Eventually it inactivates enough ribosomes that the cell dies.
Honey: One of the most calorie dense foods in nature comprised of roughly 15% water. Bees spend the summer collecting nectar, which is 30-50% sugar, and then they concentrate that nectar to roughly 75% sugar, add a few molecules of their own, et voilà: a magically sweet food that’s incredibly energy dense and yet highly inhospitable to microbes, which keeps it edible through the winter. That inhospitableness is thanks in part to its dryness.
One molecule of glucose is able to attract and mostly hold on to 21 molecules of water. And the key here is: the more strongly all those sugars in honey hold on to water, the less likely that microbes are to pry those water molecules away, so the less able they are to grow.
Aphids
Feeding: Aphids spit out a small bead of saliva that quickly hardens to the consistency of peanut butter. The aphid pushes its stylet into the plant in gentle pulses: before each pulse, she spits out a small glob of gel saliva, then penetrates it, and when the tip of her stylet pokes out the other side of the glob, she spits out another glob, penetrates that one until her stylet tip comes out the other side, and so on. These globs of gel saliva harden, creating a sheath that protects (and lubricates) her stylet as she pushes it between plant cells, farther into the plant. Once inside, the aphid takes a “sip” of the cell’s contents. Eventually the aphid penetrates the holy grail of plant anatomy, the sugar superhighway that is a sieve tube.
Aphid poop is not like your poop. Chemically it’s not all that different from sap; it’s a clear and colorless sweet, syrupy liquid. You might already know it by a different name: honeydew.
_____________________________________________________________________________
Misc Quotes
Threshold for Truth: the mental line an idea must cross before it’s accepted it as fact.
Polar Molecules: Molecules which behave as though they were embedded with tiny magnets, usually more than one per molecule.
Telescoping Generations: Offspring born identical to the parent and already pregnant with another clone.
Pickle: A cucumber “embalmed” in a mixture of water, acetic acid, and salt.
_____________________________________________________________________________
Chronology
2019: Hawaii bans both oxybenzone and octinoxate chemicals in sunscreens.-Ingredients by Zaidan.
2013: The Prevención con Dieta Mediterránea (PREDIMED) study was published; PREDIMED was a huge long-term randomized controlled trial with almost 8,000 participants followed up for five years. The main headline finding, was that people who ate a Mediterranean diet supplemented with olive oil or nuts had a roughly 30% lower risk of major cardiovascular events. Instead of randomizing people they randomized clinics. In other words, instead of splitting everyone in the village into two groups—the regular diet group and the Mediterranean diet group—they just threw everyone in each clinic into the same group. if you don’t randomize those people, you can artificially inflate or deflate the effectiveness of whatever drug, diet, or other intervention you’re testing.* PREDIMED’s mistake was discovered five years after the study was initially published.-Ingredients by Zaidan.
2012: The US FDA’s sunscreen labeling rules go into effect.-Ingredients by Zaidan.
2005: “Why Most Published Research Findings Are False” is published by Greek epidemiologist John Loannidis.-Ingredients by Zaidan.
2003: The modern e-cigarette is invented by Chinese pharmacist Hon Like, who wanted to develop something that both delivered nicotine and also preserved the ritual, because he felt that was the best way to switch from regular cigarettes to something less harmful.-Ingredients by Zaidan.
1968: Scientists discover that XP is caused by inherited mutations in a few key genes your body needs to repair its DNA.-Ingredients by Zaidan.
1935: Ambre Solaire, modern sunscreen, is invented by Eugène Schueller.-Ingredients by Zaidan.
1788: The British begin shipping convicts to Australia; by 1868, they had sent over 150,000.-Ingredients by Zaidan.
1747: The Link between Scurvy and Vitamin C is discovered aboard the British Naval vessel HMS Salisbury by James Lind, who ran a controlled trial on 12 sailors with scurvy. Lind split them up into six groups of two. Each group got a different potential cure: a quart of hard cider, 75 drops of sulfuric acid, two spoonful’s of vinegar, half a pint of seawater, the bigness of a nutmeg, or two oranges and a lemon. The sailors given oranges and lemons made a full recovery in six days. Those given the cider got a bit better. The rest stayed the same.-Ingredients by Zaidan.
1c: Humans discern how to crystallize sugar from sugarcane.-Ingredients by Zaidan.
_____________________________________________________________________________